Describe How Carbon-14 Is Used in Radiocarbon Dating
Radiocarbon dating or carbon-14 dating can be used to date material that had its origins in a living thing as long as the material contains carbon. Carbon-14 dating also called radiocarbon dating method of age determination that depends upon the decay to nitrogen of radiocarbon carbon-14.
Radiocarbon Dating Background Anu Research School Of Earth Sciences
Every 5730 years the radioactivity of carbon-14 decays by half.
. This is not as clear-cut as it seems as the amount of 14 C isotopes in the atmosphere can vary. Plants and other autotrophs take in carbon dioxide gas from the atmosphere during photosynthesis. Radiocarbon carbon-14 is a very unstable element that quickly changes into nitrogen.
Radiocarbon dating relies on the carbon isotopes carbon-14 and carbon-12. What methods do they use and how do these methods work. Carbon-14 dating known also as radiocarbon dating is a method for determining the age of an object containing organic material by using the properties of radionuclide carbon-14.
Traditional radiocarbon dating is applied to organic remains between 500 and 50000 years old and exploits the fact that trace amounts of radioactive carbon are found in the natural environment. This article reports on the use of digital image processing techniques to analyze an ultraviolet-fluorescence photograph of the area of the Shroud of Turin where the radiocarbon dating samples were taken. This is not does clear-cut as it seems as the amount.
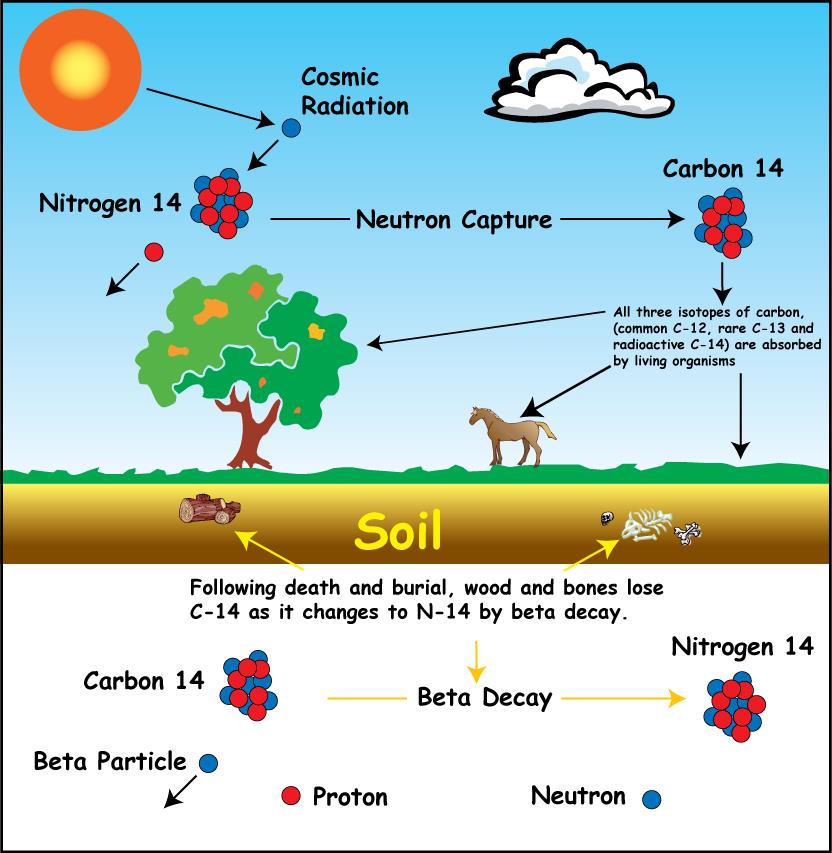
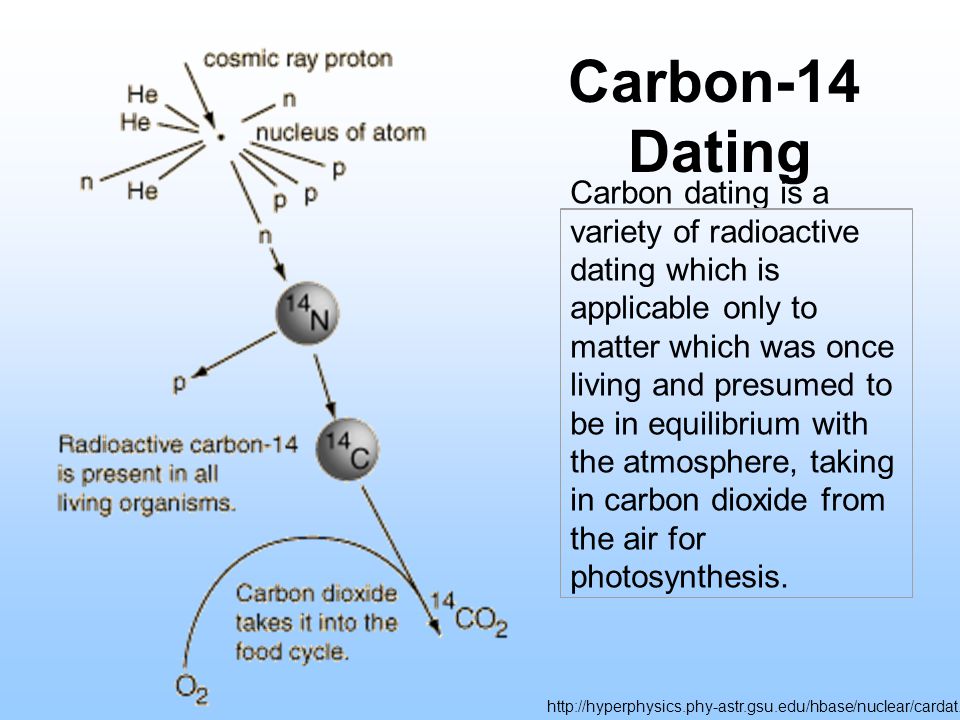
That half-life is critical to radiocarbon dating. Half the original quantity of carbon-14 will decay back to. The neutrons required for this reaction are produced by cosmic rays interacting with the atmosphere.
On the other hand carbon-14 is radioactive and decays into nitrogen-14 over time. Radioactive carbon-14 has a half-life of 5730 years and undergoes β decay where the neutron is converted into a proton an electron and an electron antineutrino. It is used in dating.
Radiocarbon dating is simply a measure of the level of 14 C isotope within the organic remains 8. Its presence in organic materials is the basis of the radiocarbon dating method pioneered by Willard Libby and colleagues 1949 to date archaeological geological and hydrogeological samples. The techniques employed in this investigation are.
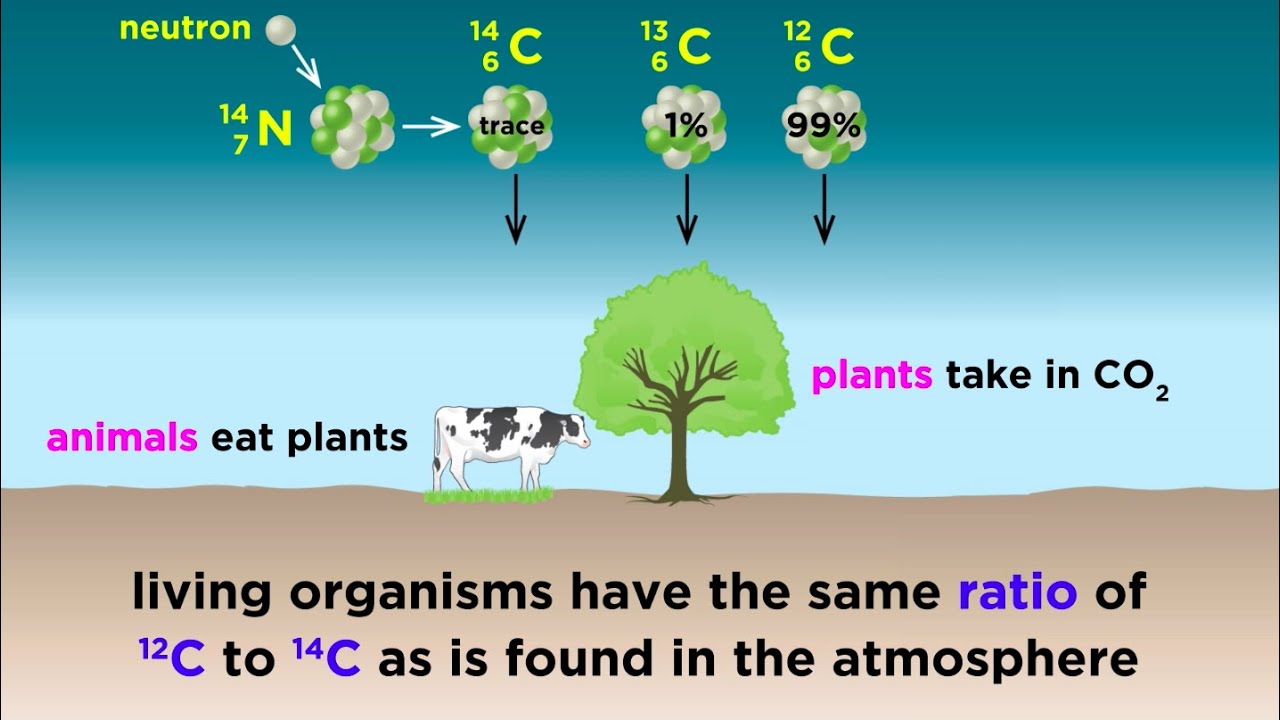
Archaeologists have long used carbon-14 dating also known as radiocarbon dating to estimate the age of certain objects. The answer is a matter of basic physics. If the level is constant living plants and animals should also maintain a constant carbon-14 level in them.
Carbon-14 is a carbon isotope that is commonly used by. The method was developed in the late 1940s at the University of Chicago by Willard Libby. In this article we will examine the methods by which scientists use radioactivity to determine the age of objects most notably carbon-14 dating.
Beta decay of C-14. C-14 carbon dating process. By comparing this with a modern standard an estimate of the calendar age of the artefact can be made.
The radiocarbon half- life or decay rate has been determined at 5730 years. This is why calibration against objects whose age is known is required 14. Scientists are looking for the ratio of.
Some materials that do not contain carbon like clay pots can be dated if they were fired in an oven. Carbon-14 dating is a way of determining the age of certain archeological artifacts of a biological origin up to about 50000 years old. If carbon- 14 has formed at a constant rate for a very long time and continually mixed into the biosphere then the level of carbon-14 should remain constant.
It is often used in archeology and some types of biology. Living creatures ingest carbon. Radiocarbon dating is the use of the naturally occurring isotope of carbon -14 in radiometric dating to determine the age of organic materials up to ca.
Radiocarbon dating is simply a measure of the level of 14 C isotope within the organic remains 8. Carbon -14 is continually formed in nature by the interaction of neutrons with nitrogen-14 in the Earths atmosphere. Carbon-14 14 C or radiocarbon is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons.
Carbon dating also referred to as radiocarbon dating or carbon-14 dating is a method that is used to determine the age of an object. When an organism dies it carbon radiocarbon the dummies isotope and c-14 starts decaying 7. Carbon-14 dating also called radiocarbon dating is used to determine the age of materials that contain carbon that was originally in living things.
Historical artefacts like moa bones can be dated using a technique that measures the activity of the radioisotope carbon-14 still present in the sample. Radiocarbon dating uses carbon isotopes. Within archaeology it is considered an absolute dating technique.
To use this interactive move your mouse or finger over any of the labelled boxes and click to obtain. Radiocarbon dating also referred to as carbon dating or carbon-14 dating is a method for determining the age of an object containing organic material by using the properties of radiocarbon a radioactive isotope of carbon. Why Isnt Carbon Dating Used to Date Fossils.
Results of this analysis demonstrate the anomalous nature of the radiocarbon data sample area.

What Is Carbon 14 14c Dating Carbon Dating Definition

Carbon 14 Dating Carbon Dating Is A Variety Of Radioactive Dating Which Is Applicable Only To Matter Which Was Once Living And Presumed To Be In Equilibrium Ppt Video Online Download
Uses Of Radioactivity Radiocarbon Dating Pass My Exams Easy Exam Revision Notes For Gsce Physics
Comments
Post a Comment